








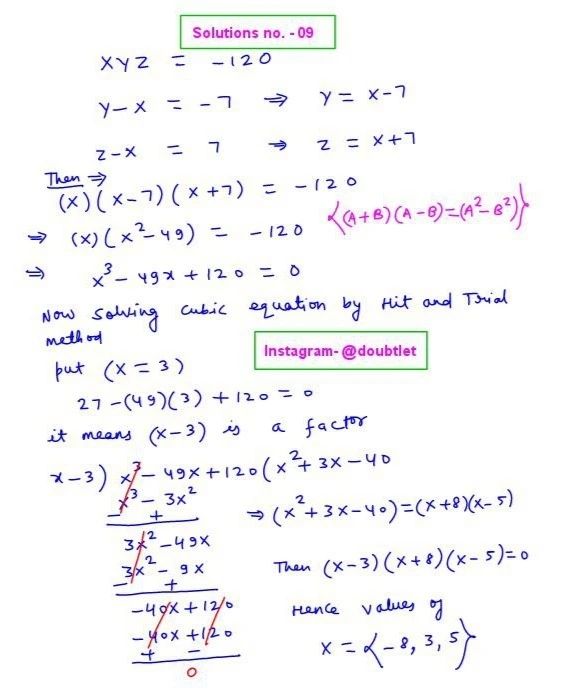
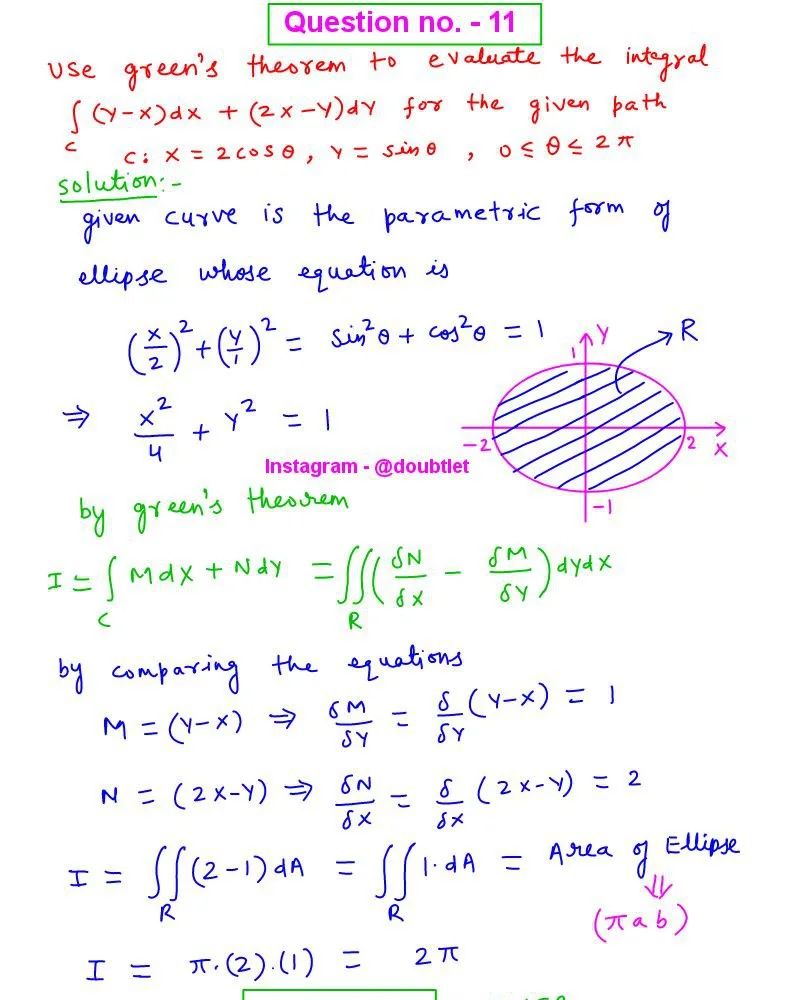
Question :
Solution:

Neetesh Kumar | September 05, 2024
This is the solution to Myopenmath Math2A Differential Equation
** Growth and Decay** homework question number 4.1.3
Contact me if you need help with homework, assignments, or Exams for STEM subjects.
You can see our Testimonials or Vouches from here of the previous works I have done.
Step-by-Step-Solution:
We are given that a radioactive material loses 34% of its mass in 8 minutes. We need to find its half-life.
The exponential decay model is given by the equation:
Where:
- is the quantity of material remaining after time ,
- is the initial quantity of the material,
- is the decay constant,
- is the time.
Step 1: Relating the Given Information
We are told that 34% of the material is lost, meaning 66% remains. This can be expressed as:
We are also told that this occurs at minutes. Substituting this into the exponential decay equation:
Step 2: Simplifying the Equation
Cancel out from both sides:
Step 3: Taking the Natural Logarithm of Both Sides
Take the natural logarithm of both sides to solve for :
Step 4: Solving for the Decay Constant
Now solve for :
Step 5: Finding the Half-Life
The half-life is related to the decay constant by the equation:
Solving for the half-life :
Substitute the value of :
Thus, the half-life of the material is
approximately minutes.
If this solution helps, then please share this with your friends.
Please subscribe to my Youtube channel for video solutions to similar questions.
Keep Smiling :-)
Comments(0)
Leave a comment